Bio-engineering
Proteomic Study of Inner Ear
Research on the ear faces significant challenges due to its small sensory structures being encased in large fluid-filled spaces and a dense bone shell. Moreover, diagnosing and treating inner ear disorders is difficult for several reasons: 1) many pathological inner ear conditions have unknown causes, 2) there is a lack of precise and accurate methods for analyzing the inner ear, and 3) there are no specific biomarkers for inner ear diseases to indicate their cause, severity, and progression. Therefore, identifying biomarkers for early diagnosis is crucial for providing real-time information and guiding diagnostic and treatment decisions for inner ear disorders. Additionally, this can offer new insights into the underlying mechanisms of these diseases.
Proteomics has become a key approach for identifying the total set of proteins in organisms, as well as detecting both qualitative and quantitative changes in proteins in response to different conditions. In collaboration with the Australian Proteome Analysis Facility (APAF), we aim to construct an in-depth whole proteome of inner ear fluid and cochlea tissue in both animal models and human to determine the mechanism of hearing loss and potential biomarkers in humans.
Research on the ear faces significant challenges due to its small sensory structures being encased in large fluid-filled spaces and a dense bone shell. Moreover, diagnosing and treating inner ear disorders is difficult for several reasons: 1) many pathological inner ear conditions have unknown causes, 2) there is a lack of precise and accurate methods for analyzing the inner ear, and 3) there are no specific biomarkers for inner ear diseases to indicate their cause, severity, and progression. Therefore, identifying biomarkers for early diagnosis is crucial for providing real-time information and guiding diagnostic and treatment decisions for inner ear disorders. Additionally, this can offer new insights into the underlying mechanisms of these diseases.
Proteomics has become a key approach for identifying the total set of proteins in organisms, as well as detecting both qualitative and quantitative changes in proteins in response to different conditions. In collaboration with the Australian Proteome Analysis Facility (APAF), we aim to construct an in-depth whole proteome of inner ear fluid and cochlea tissue in both animal models and human to determine the mechanism of hearing loss and potential biomarkers in humans.
Microfluidics Cell Sorting
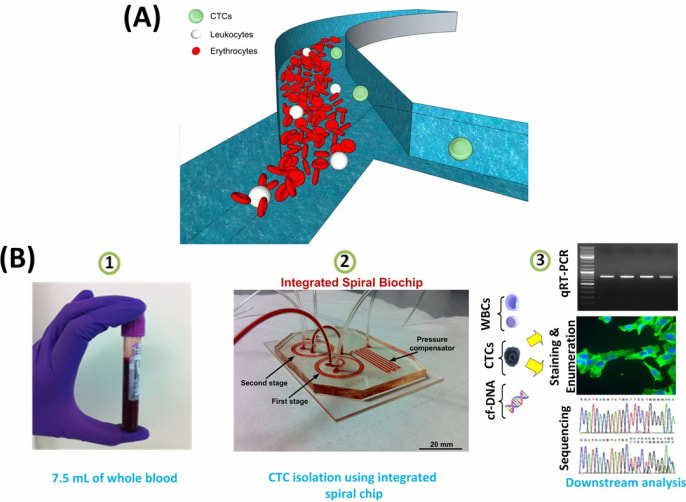
In collaboration with Dr Warkiani group in UTS, we aim to develop novel microfludic devices for various applications including cell sorting. Cell sorting is critical for many applications ranging from stem cell research to cancer therapy. Isolation and fractionation of cells using microfluidic platforms have been flourishing areas of development in recent years. The need for efficient and high-throughput cell enrichment, which is an essential preparatory step in many chemical and biological assays, has led to the recent development of numerous microscale separation techniques. We have pioneered some inertial microfluidic platforms for various applications, including separation of circulating tumor cells (CTCs) from blood, enrichment of malaria parasite and fractionation of mesenchymal stem cells (MSCs).
In collaboration with Dr Warkiani group in UTS, we aim to develop novel microfludic devices for various applications including cell sorting. Cell sorting is critical for many applications ranging from stem cell research to cancer therapy. Isolation and fractionation of cells using microfluidic platforms have been flourishing areas of development in recent years. The need for efficient and high-throughput cell enrichment, which is an essential preparatory step in many chemical and biological assays, has led to the recent development of numerous microscale separation techniques. We have pioneered some inertial microfluidic platforms for various applications, including separation of circulating tumor cells (CTCs) from blood, enrichment of malaria parasite and fractionation of mesenchymal stem cells (MSCs).